Fluorographite Nanoplatelets with Covalent Grafting of Anion-Exchange Resins for Water Purification
Abstract
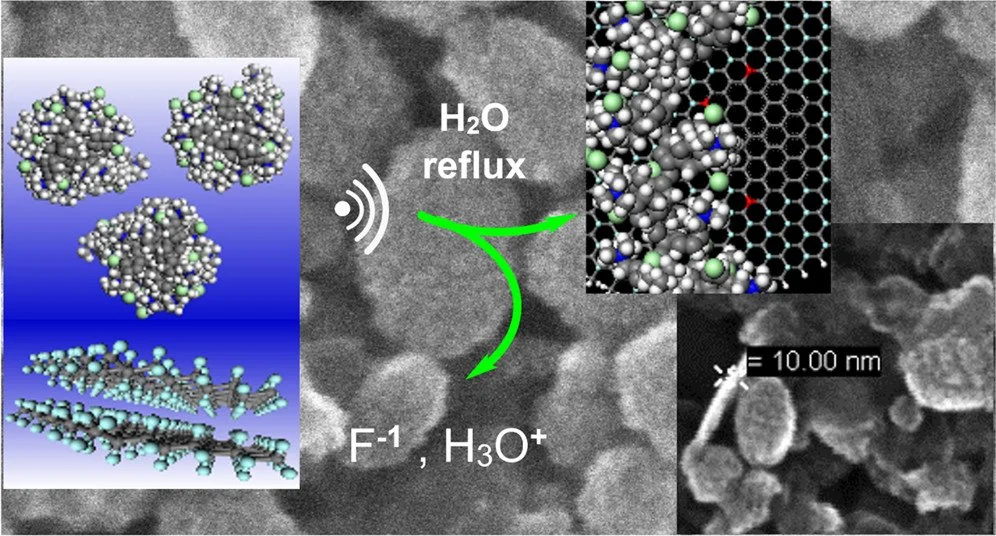
We have developed a novel green synthetic method to covalently graft fluorographite (FGi) nanoplatelets, with quaternary ammonium polyelectrolyte chains under mild reaction conditions in water. Radical centers on the fluorographite layers react with the radical chain end on short strands of anion-exchange resins. While fluorographite is superhydrophobic, we show that the polymer radical chain end is necessary to initiate defluorination and delamination of the FGi in neutral pH water, without any pretreatment or caustic reagents. Scanning electron microscopy of thin films shows continuous and organized stacking of ellipsoidal nanoplatelets across large defect-free areas. We show that these new materials are highly effective at removing known and emerging contaminants to below environmentally relevant concentrations. Electron microscopy, vibrational spectroscopy, elemental analysis, and thermal analysis data are presented, and they are consistent with defluorination, partial exfoliation, and graphitization during the aqueous polymer grafting reaction. A radical-initiated mechanism is proposed that is consistent with the observed defluorination and oxidation of FGi nanoplatelets. The physicochemical properties, water flux, and morphology of these thin-film assemblies are described in detail. Thin membranes of polymer-functionalized fluorographite removed 99% of perfluorooctanoic acid to below 100 parts per trillion while maintaining a very high water flux over 1100 L h–1 m–2 bar–1. Percent removal of perfluorinated alkyl substances and heavy metal oxyanions versus polyelectrolyte-functionalized fluorographite membrane areal density is reported. The methodology presented in this study is a facile approach toward developing high-performance materials for sustainable and green applications.